Breast Cancer and Menopause
Key Points
|
![]() AMS Breast Cancer and Menopause 146.46 KB
AMS Breast Cancer and Menopause 146.46 KB
Introduction
Menopause/menopausal symptoms in women with breast cancer (BC) have a significant negative impact on quality of life, with both short- and long-term health consequences, and can affect BC treatment adherence. Menopause/menopausal symptoms in women with BC may be associated with (1) natural menopause occurring concurrently with a BC diagnosis, (2) recurrence of menopausal symptoms following cessation of menopausal hormone therapy (MHT) upon breast cancer diagnosis, (3) risk-reducing bilateral oophorectomy, chemotherapy or ovarian suppression secondary to gonadotropin releasing hormone (GnRH) analogues in premenopausal women, and/or (4) endocrine adjuvant therapy with tamoxifen or aromatase inhibitors1.
Women with breast cancer, especially younger women, experience more severe menopausal symptoms than women without breast cancer2 . The spectrum of symptoms is similar to usual age menopause (See AMS information sheet What is menopause?) and a study of 843 Australian women indicated that most women aged 50-69 years had persisting vasomotor, psychosocial, physical and sexual symptoms at 6 years post BC diagnosis despite having ceased adjuvant endocrine therapy3.
Women with BC may be at increased risk of cardiovascular disease (CVD). A systematic review assessing CVD mortality in women with BC reported that 9.4-10.4% died of CVD compared to 7.4-7.5% women without BC4. Multiple factors may influence CVD risk including the indirect/ direct effects of therapies including chemotherapy, radiotherapy, adjuvant endocrine therapies, lifestyle changes and weight gain accompanying BC diagnosis. The ten-year predicted CVD risk was greater than or equal to BC risk recurrence in a study of 415 women with BC, mean age 60 years5. Osteoporosis and fracture risk is also increased in women with BC, the greatest bone loss observed in younger women treated with GnRH analogue + aromatase inhibitor6. Adjuvant endocrine therapy, vitamin D deficiency and increased falls risk secondary to chemotherapy-induced neuropathy are some of the factors which contribute to osteoporosis risk6. Oestrogen depletion due to aromatase inhibitors is associated with accelerated bone loss which predisposes to increased fracture risk. In contrast, tamoxifen in postmenopausal women acts as an oestrogen on bone and retards bone resorption and reduces fracture risk.
Management of menopause in women with breast cancer is directed at relieving troublesome symptoms and minimising risks of cardiovascular disease, osteoporosis and breast cancer recurrence.
Diagnosis of menopause
The diagnosis of menopause is obvious after bilateral oophorectomy or where menopause occurred prior to BC diagnosis. However, in premenopausal women the diagnosis of menopause following BC chemotherapy can be difficult especially with concurrent tamoxifen therapy. FSH levels can be difficult to interpret in the women receiving tamoxifen, since, in contrast to its action in premenopausal women, tamoxifen partially suppresses gonadotrophins in the postmenopausal woman7. A model combining anti-mullerian hormone levels with age has been proposed to assist with the prediction of amenorrhoea post-chemotherapy8; however, validation in the real world setting is lacking.
Management of Menopause
-
Vasomotor symptoms
Although oestrogen containing MHT is the most effective therapy for vasomotor symptoms, there is conflicting evidence regarding safety of MHT after BC and it is not recommended, regardless of the hormone receptor status of the tumour. Clinical trials of MHT in women with early BC indicated that BC recurrence was increased after 2 years average follow-up in the HABITS study9 (Relative hazard [RH] = 3.3, 95% confidence interval [CI] = 1.5 to 7.4) but not after 4 years of follow-up in the Stockholm study10 (RH = 0.82, 95% CI = 0.35 to 1.9). These differences may relate to differences in progestogen exposure (continuous in HABIT and sequentially every 1-3 months in Stockholm), less tamoxifen use and higher rate of node positive patients in the HABITS trial10. Results from the LIBERATE trial indicated an increased risk of BC recurrence with tibolone therapy BC survivors (overall RR 1.40 95%CI 1.14-1.70)11 regardless of whether the tumour was oestrogen and/or progesterone receptor positive. Although, the recurrence risk may be less in women with oestrogen receptor negative cancer11. Historically, high dose progestogen therapy was used as adjuvant therapy. As MHT is not generally recommended, a variety of non-hormonal therapies for vasomotor symptoms have been investigated (see AMS information sheets Nonhormonal treatments for menopausal symptoms and Complementary and herbal therapies for hot flushes). Findings from a recent comparative analysis12 are shown in Figure 1, indicating that the non-hormonal SSRIs/ SNRIs and neuroleptic agents (including gabapentin and pregabalin) had the greatest efficacy; whereas botanicals, magnesium and Vitamin E were comparable to placebo. These findings are consistent with recommendations of the North American Menopause Society (NAMS) position statement which also recommended cognitive behavioural therapy and hypnosis as treatment options (http://www.menopause.org/docs/default-source/professional/2015-nonhormonal-therapy-position-statement.pdf). The safety of botanicals and phytoestrogens in BC survivors is unclear. Compounded bioidentical hormone preparations may contain oestrogens and progestogens and their safety in BC is unknown.
-
Urogenital symptoms
Up to 75% of women with BC report at least one urogenital symptom if asked; however, many women do not raise this issue. Urogenital symptoms were considered to have the highest prevalence of “unmet need”13. Chemotherapy, aromatase inhibitor treatment and smoking are risk factors for urogenital symptoms. Vaginal oestrogens are the most effective treatment for urogenital symptoms; however, the use of these agents by women with BC, especially those taking aromatase inhibitors, is controversial. Recommendations from NAMS (https://www.menopause.org/docs/default-source/professional/management_of_genitourinary_syndrome_of_menopause.pdf) and the American College of Obstetricians and Gynaecologists (https://www.acog.org/-/media/Committee-Opinions/Committee-on-Gynecologic-Practice/co659.pdf?dmc=1&ts=20190527T0539259779) regarding management are:
(a) comprehensive assessment including history and physical examination
(b) counselling with validation and explanation of symptoms
(c) Non-hormonal moisturisers and lubricants, pelvic floor physiotherapy and dilator
therapy are first line agents
(d) Long term safety and efficacy data are lacking for laser therapy (See AMS fact
sheet Vaginal laser therapy)
(e) For women taking adjuvant endocrine therapy who have an inadequate response
with non-hormonal agents, discussion with the women, her oncologist and an
individualised risk assessment is required for consideration regarding use of vaginal
oestrogen. The use of intravaginal oestrogens with tamoxifen is of less concern
compared to that in women taking aromatase inhibitors.
The safety of ospemifene, intravaginal DHEA and intravaginal testosterone is unknown in women with BC.
-
Lifestyle
Weight gain of 2.3-5kg occurs following BC diagnosis/ treatment and is multifactorial14. Excess weight is associated with increased BC recurrence. In overweight women, weight loss may assist with control of vasomotor symptoms and reduce CVD risk (see AMS information sheet Weight management and healthy ageing).
-
Bone health
Assessment of fracture risk is important for all women diagnosed with BC due to the negative impact of cancer therapies on bone health. A position statement6 , co-authored by the Australasian Menopause Society, recommends a comprehensive assessment of bone health including history to identify additional risk factors and investigations including biochemistry (serum electrolytes, liver function, 25OH Vitamin D, TSH, calcium, magnesium and phosphate), bone densitometry (DXA) and plain thoracolumbar Xrays (or VFA as part of DXA). Management should be directed at optimising dietary calcium intake (1000-1200mg/day), weight bearing exercise and vitamin D. Anti-resorptive therapy may be required in the setting of (i) prevalent or incident fragility fracture (ii) T score < -2.0 or significant bone loss.
-
Contraception
Pregnancy should be avoided during active treatment for BC. Systemic hormonal contraception (including oral, vaginal rings, subdermal or intrauterine) is not recommended15. The copper T IUD is recommended as a safe and effective form of contraception for women with BC. IUDs are 99.2% effective and provide contraception for 10 years15. Barrier and natural methods can be used although are less effective.
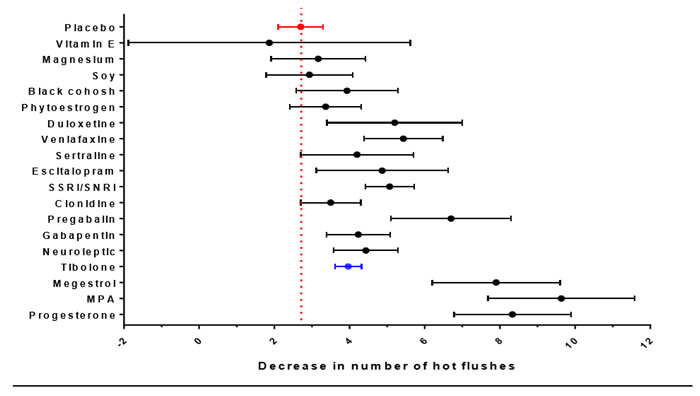
Figure 1: Calculated comparative efficacy (with 95% confidence interval) in reduction of hot flush frequency. Red dotted line indicates calculated placebo value and blue point indicates tibolone. Data derived from12.
References
- Vincent AJ. Management of menopause in women with breast cancer. Climacteric. 2015;18(5):690-701.
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of Life, Fertility Concerns, and Behavioral Health Outcomes in Younger Breast Cancer Survivors: A Systematic Review. Journal of the National Cancer Institute. 2012;104(5):386-405.
- Davis SR, Panjari M, Robinson PJ, Fradkin P, Bell RJ. Menopausal symptoms in breast cancer survivors nearly 6 years after diagnosis. Menopause. 2014;21(10):1075-1081 1010.1097/GME.0000000000000219.
- Gernaat SAM, Ho PJ, Rijnberg N, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Research and Treatment. 2017;164(3):537-555.
- Bardia A, Arieas ET, Zhang Z, et al. Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer. Breast Cancer Res Treat. 2012;131(3):907-914.
- Grossmann M, Ramchand SK, Milat F, et al. Assessment and management of bone health in women with oestrogen receptor‐positive breast cancer receiving endocrine therapy: Position statement of the Endocrine Society of Australia, the Australian and New Zealand Bone & Mineral Society, the Australasian Menopause Society and the Clinical Oncology Society of Australia. Clinical Endocrinology. 2018;89(3):280-296.
- Lonning PE, Johannessen DC, Lien EA, Ekse D, Fotsis T, Adlercreutz H. Influence of tamoxifen on sex hormones, gonadotrophins and sex hormone binding globulin in postmenopausal breast cancer patients. J Steroid Biochem Mol Biol. 1995;52(5):491-496.
- Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Human Reproduction Update. 2014;20(3):370-385.
- Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer--is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363(9407):453-455.
- von Schoultz E, Rutqvist LE, Stockholm Breast Cancer Study G. Menopausal hormone therapy after breast cancer: the Stockholm randomized trial.[see comment]. Journal of the National Cancer Institute97(7):533-5. 2005.
- Kenemans P, Bundred NJ, Foidart JM, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol. 2009;10(2):135-146.
- Li T, Yang J, Lv Y, et al. Quantitative comparison of drug efficacy in treating hot flashes in patients with breast cancer. Breast Cancer Research and Treatment. 2019;173(3):511-520.
- Yoon J, Malin JL, Tao ML, et al. Symptoms after breast cancer treatment: Are they influenced by patient characteristics? Breast Cancer Research and Treatment. 2008;108(2):153-165.
- Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: Prevalence, pattern and health consequences. Obesity Reviews. 2011;12(4):282-294.
- WHO. Medical eligibility criteria for contraceptive use Fifth ed. 20 Avenue Appia, 1211 Geneva 27, Switzerland: WHO Press, World Health Organization,; 2015.

Note: Medical and scientific information provided and endorsed by the Australasian Menopause Society might not be relevant to a particular person's circumstances and should always be discussed with that person's own healthcare provider. This Information Sheet may contain copyright or otherwise protected material. Reproduction of this Information Sheet by Australasian Menopause Society Members and other health professionals for clinical practice is permissible. Any other use of this information (hardcopy and electronic versions) must be agreed to and approved by the Australasian Menopause Society.
Content updated September 2019
